The
following copyrighted material is intended for individual use of the
researcher, and may not be copied or distributed without written permission
from the copyright holder. You may use it for non-profit scholarly purposes.
The reference for this article is: Rivas, J. A. and Burghardt G. M. 2001
Sexual size dimorphism in snakes: wearing the snake�s shoes. Animal Behaviour.
62(3): F1-F6. Click here for a pdf version of the
file
UNDERSTANDING SEXUAL SIZE DIMORPHISM IN SNAKES: WEARING THE SNAKE’S SHOES
Sexual size dimorphism (SSD) is widespread in almost every group of animals, generating great scientific interest (Andersson 1994). This is particularly true in snakes, in which intraspecific aggression, dominance hierarchies, and territoriality are rare. On the other hand, SSD in snakes is often both extreme and female biased. If males fight with each other and winners obtain multiple matins, males should evolve larger sizes (Shine 1978; Shine 1993; Madsen & Shine 1994). There is, however, some evidence that this is not always the case (Madsen et al. 1993; Madsen & Shine 1993; Weatherhead et al. 1995). Recently Shine et al. (2000) and Crews (2000) discussed conflicting evidence on the role of male body size in mating success in garter snakes (Thamnophis sirtalis) in this forum, but were unable to resolve the different empirical results. Sometimes scientific assumptions or from ingrained points of view may hinder recognition of other possibilities. Here we here discuss an alternative approach to generating testable hypotheses: critical anthropomorphism (Burghardt 1991). By using critical anthropomorphism we propose a new hypothesis that has the potential to settle this controversy.
Jacob von Uexk�ll (1909/1985) advocated studying the behaviour of animals by considering both their inner world (Innenwelt) and how they perceived and responded to their environment (Umwelt). A major aspect of this approach was to evaluate differences among species in the salience of biologically relevant perceptual cues (Tinbergen 1951; Burghardt 1985). Recent proposals to study animal cognition focus on the ways that animals perceive, interpret, and experience the world (Griffin 1978; Cheney & Seyfarth 1992; Glotzbach 1992; Burghardt 1997; Bekoff & Allen 1997). An important component of this approach, though often understated, is to consider the animal being studied as an active participant, with the researcher trying to put him or herself in the animal's situation. This is especially true for those studying primate behaviour (Herzog & Galvin 1997). Timberlake & Delamater (1991) proposed that to understand the behavior of an animal "Experimenters not only need to put themselves in the subject�s shoes, they need to wear them - walk, watch, hear, touch, and act like the subject" (p: 39). One approach to doing this and still maintaining scientific rigor is to apply a critical anthropomorphism in which hypotheses are based on scientific knowledge about the species being studied as well as our experiential projection into the "shoes" (point of view) of others (Burghardt 1991). We apply this method to the maintenance of female biased SSD in snakes, where males compete physically for access to females in the context of actual mating.
In most snake species females are larger than males, reversing the typical terrestrial vertebrate pattern where males are of equivalent or larger size than females (Shine 1994). Large size in female snakes is considered adaptive in species that grow throughout life with little or no parental care, and in which larger females produce more and/or larger offspring. Larger offspring have higher survival rates and can store more yolk or fat for their development (see Ford & Seigel 1989 for a review). Natural selection should, therefore, favour large size in females. Male snakes, on the other hand, benefit from traits that enhance their ability to find and successfully court females (Table 1). Thus, refined chemosensory senses, high mobility, being inconspicuous to predators, early maturation, small size, and decreased costs of locomotion would be adaptive (Duvall et al. 1993; Shine 1993; Madsen et al. 1993; Andersson 1994).
Female biased SSD is probably the ancestral condition of snakes as a group (Rivas 1999). Thus, smaller size in males can be explained by the lack of selection pressure toward large body size (Semlitsch & Gibbons 1982). Unlike lizards, their sister squamate taxa, territoriality has not been reported in snakes, and male-male fighting is also uncommon. Thus, selection forces for large male size are generally lacking (Shine 1993). However, in conditions of high density, where females are very easy to track, or where females do not breed every year, several males would encounter each other while courting a female and male-male combat is likely to evolve (Duvall et al. 1992; Shine 1978, 1993). The relationship between male-male fighting and male size in snakes has been discussed broadly (Shine 1978; Madsen et al. 1993; Shine 1993; Madsen & Shine 1994). If larger males are more successful in combat and obtain more matings than smaller males, large size in males would be favoured by selection. Male combat is much more frequent in snake species where males are larger, or where SSD is absent (Shine 1994).
There are, however, some species where sexual selection favours larger males, yet males are not larger than females (Madsen et al. 1993; Madsen & Shine 1993; Weatherhead et al. 1995). Grass snakes (Natrix natrix) breed in mating balls where males wrestle with the tail in subtle combat and larger males obtain more matings than small males (Madsen & Shine 1993). A similar scenario has been found in northern water snake (Nerodia sipedon) where larger males accomplish more matings in multiple-male breeding aggregations (Weatherhead et al 1995; Brown & Weatherhead 1999). On the other hand, there are data showing that male common garter snakes, Thamnophis sirtalis, do not obtain an advantage by being larger (Joy & Crews 1988). However, recent studies on the same population reveal that larger male garter snakes do obtain more matings than smaller males (Shine et al. 2000). Madsen and Shine (1993) argue that if females obtain more benefits from large size than do males; males may remain smaller than females. However, the selection gradient based on increased size for males obtaining multiple matings is higher than the comparable selection gradient for females due to increased fecundity by being larger (Duvall et al. 1993). In addition, any increase in the fitness of a female for being larger than others (and thus having larger clutches) would also increase the reproductive output of a male that mates successfully with her, thus increasing the benefit for the male as well. Larger males might also be more successful in mating multiple times within a season (Madsen et al 1993; Shine and Fitzgerald 1995; Weatherhead et al. 1995). The selection gradient for males is proportional to the fecundity of females (Duvall et al 1993). Thus, it is not clear how an increase in fecundity of larger females would not increase even more the fitness of males that can obtain more matings in the polygynous system considered the dominant mating system in snakes (Duvall et al 1992, 1993; but see Rivas 1999). Furthermore, homologous morphological traits in males and females are expected to show high genetic correlations (Halliday & Arnold 1987); if true, any selection for large size in females should also increase the size of the males. Madsen and Shine (1994) further argue that sex differences in costs of reproduction can affect the optimal body size of each sex and produce the SSD found in European adders (Vipera berus). Predictions from these models do not appear plausible when faced with the extent of the dimorphism seen. Perhaps we have been overly influenced by the behaviour of lizards, birds, and mammals, in which size and strength seem to be major determinants in mating success, and have inappropriately applied the evolutionary logic proposed for these groups to snakes.
In the literature regarding SSD it has not
been hitherto proposed that males could suffer a sexual selection disadvantage
from being too large. Consider the problems of being a male snake in search of
potential mates, putting ourselves in the "animals shoes". Being too
large could actually be a disadvantage in multi-male breeding aggregations. Males
search with their tails for the female�s cloaca (as described by
To seek and court large bulkier animals is adaptive for a male, since larger and thicker females have more offspring (Ford and Seigel 1989) and are more likely to breed (Rivas 1999). Such females are also older and more experienced. Hence, it would benefit males to court the animals with largest girth, both for certainty of courting the right sex (and individual) and for increase of fitness. Thus, SSD could be the key for sex identification in situations where the chemosensory organs are not involved or the pheromones of the females and scents of the males have impregnated all the animals in the seething breeding ball. Success may belong to the male who can best discriminate males from females, manoeuvre into position for copulation, and simultaneously thwart other males from doing likewise.
The above scenario can be applied and
partially tested by studies of green anacondas (Eunectes murinus), which
also breed in multiple-male aggregations where a female is courted by several
males. In these aggregations males coil around the female and search for her
cloaca with their tails, visual or chemical cues did not seem to be involved
(Figure 1; Rivas 1999). If one male is very large it can be mistaken for a female
by other males and be courted (Figure 2). Selection would favor large size in
males in order to outcompete other males, as larger males are more likely to be
found mating with the larger and more fecund females (Rivas 1999). However,
there is an optimal size where males start being confused with females by other
males; this imposes a limit on male size. The result would be stabilising
selection on males, producing a population structure where all the males have a
very similar adult size and the overlap of size between males and females is
minimal (Figure 3). This sort of confusion between large males and females that
we predict could be present in most, if not all multi-male breeding
aggregations. In particular it seems to apply to the experiment of Madsen &
Shine (1993) with grass snakes (Natrix natrix), where they report that
"Males seemed to become confused between the female's tail and those of
other males, and the tails of rival males often became entwined" (p: 562.
That size is used as a cue by males to female presence is also suggested in the
report of Noble (1937) on two small male Thamnophis sirtalis that, for
half an hour, courted a large male from another region where the animals were
not reproductively active at the time. In his recent reply to Shine et al.
(2000), Crews (2000) argued that the number of males involved in a breeding
aggregation may lead to different outcomes in the competition between males;
this could be reason his results (Joy & Crews 1988) differ from those of
Shine et al. (2000). Shine used only a relatively small number of males
(perhaps a more common scenario in the mating system of garter snake throughout
North America) while Crews worked with larger number of males per aggregation
(simulating the scenario of the particular dense breeding aggregations found in
southern
Differential maturation between the sexes may also be involved in the evolution of this mechanism of sex identification. Females often delay sexual maturation and become relatively larger, allowing larger clutches. Males start breeding earlier and at a smaller size, increasing their reproductive output since the fecundity independent costs of reproduction are lower (Bell 1980; Madsen & Shine 1994). This differential maturation sets the scenario for natural selection to act and SSD can be selected as a method for sex discrimination.
To this point we have approached the actual courtship events from the male's perspective; the female�s perspective must also be considered. Females are known to be selective in mating aggregations. Perry-Richardson et al (1990) found that female Thamnophis marcianus rejected some males, even after intromission had occurred. In breeding several generations of Thamnophis melanogaster in our laboratory, we also have noted females accepting some males and not others. Joy and Crews (1988) suggested some individual males may be consistently more successful than others. Female choice might make a large difference in the fitness of offspring. Drickamer et al. (2000) report that female Mus musculus mated with males they preferred and, as a result, had more fit offspring than females mated with non-preferred males. Comparable phenomena may occur in snakes. What decision processes are female snakes using to accept or reject a male's advances? In a breeding ball several males court a female at the same time. It is very likely that the only way she can discriminate and choose among the males is, again, by relying on tactile cues. Does she have the ability to differentiate from the displays given by the anterior end of the snake (typically directed at the dorsum of her neck), which tail is worthy of her favours? It may be necessary to observe a mating ball three dimensionally from the interior to better understand the processes involved.
Female ethologists have correctly emphasised the value that taking a female perspective has added to understanding social behaviour, especially in primates (Small 1993; Gowaty 1994; Cunningham & Birkhead 1997). If a von Uexk�llian approach to behaviour had been applied in the past, errors such as neglecting, presumably unconsciously, the role of females in social systems might never have occurred. Similarly, we feel that through applying a critical anthropomorphism it is possible to analyse the snake�s Umwelt, and obtain testable, and perhaps more valid, insights about both the processes involved in these events and how sexual selection might be operating.
Too often ethologists and herpetologists regard snakes and other reptiles as robot-like machines or as animals so alien from us that attempting to put ourselves into their world, even heuristically, is both useless and a scientifically dangerous conceit. On the contrary, approaching unresolved issues by considering the perceptual world and perspective of the target animal may generate testable hypotheses that were previously unconsidered. This may prove to be true in research on snake mating systems as well as on the evolution and maintenance of SSD.
ACKNOWLEDGMENTS
We thank R. Owens, M. Bealor, P. Andreadis,
and reviewers for comments on the ms. We also thank the Wildlife Conservation
Society, National Geographic Society, University of Tennessee Science Alliance,
and the National Science Foundation for financial support. We are also in debt
to The Corporaci�n Venezolana de Ganader�a and Estacion Cientifica Hato El Frio
for logistic support in the field.
REFERENCES
Andersson, M. 1994. Sexual
Selection. Princeton:
Bekoff, M. &
Allen, C. 1997. Cognitive ethology: slayers, skeptics and proponents. In: Anthropomorphism,
Anecdotes, and Animals (Ed. by R. W. Mitchell, N. S. Thompson, & H. L.
Miles), pp. 313-334.
Brown, G. P. & Weatherhead, P. J. 1999. Demography and sexual size dimorphism in northern water snakes, Nerodia sipedon.Canadian Journal of Zoology, 77, 1358-1366.
Burghardt, G. M.
(Ed.). 1985. Foundations of Comparative Ethology.
Burghardt, G. M.
1991. Cognitive ethology and critical anthropomorphism: a snake with two heads
and hognose snakes that play dead. In: Cognitive ethology: The Minds of
Other Animals (Ed. by C. A Ristau), pp. 53-90.
Burghardt, G. M. 1997. Amending Tinbergen: A fifth aim for ethology. In: Anthropomorphism, Anecdotes, and Animals (Ed. by R. W. Mitchell, N. S. Thompson, & H. L. Miles), pp. 254-276. New York: SUNY press.
Cheney, D. L. & Seyfarth, R. M. 1992. Pr�cis of How Monkeys See the World: Inside the Mind of Another Species. Behavioral and Brain Sciences, 15, 135-182.
Crews, D. 2000. Reply to Shine et al. (2000). Animal Behaviour, 59, F12.
Cunningham, E. & Birkhead, T. 1997. Female roles in perspective. Trends in Ecology and Evolution, 12, 337-339.
Drickamer, L. C., Gowaty, P. A. & Holmes, C. M. 2000. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Animal Behaviour, 59, 371-378.
Duvall, D., Arnold, S. J. & Schuett, G. W, 1992. Pitviper mating systems: ecological potential, sexual selection and microevolution. In: Biology of the pitvipers (Ed by J. A. Campbell and E. D. Brodie , Jr.), pp. 321-336. Arlington, TX: Selva.
Duvall, D., Schuett G. W. & Arnold, S. J. 1993. Ecology and evolution of snake mating systems. In: Snakes, Ecology and Behavior (Ed by R. A. Seigel & J. T. Collins), pp. 165-200. New York: McGraw-Hill.
Ford, N. B. & Seigel, R. A. 1989. Relationships among body size, clutch size, and egg size in three species of oviparous snakes. Herpetologica, 45, 75-83.
Gillingham, J. C. 1979. Reproductive behavior of the rat snakes of eastern North America, genus Elaphe. Copeia, 1979, 319-331.
Gillingham, J. C. 1987. Social behavior. In: Snakes: Ecology and Evolutionary Biology (Ed by R. A. Seigel, J. T. Collins, & S. S. Novak), pp. 184-209. New York: McGraw-Hill.
Glotzbach, P. A. 1992. Perception theory and the attribution of mental states. Behavioral and Brain Sciences, 15, 157-158.
Gowaty, P. A. 1994. Architects of sperm competition. Trends in Ecology and Evolution, 9, 160-162.
Griffin, D. R. 1978. Prospects for a cognitive ethology. Behavioral and Brain Sciences, 1, 527-538.
Halliday T. & Arnold, S. J. 1987. Multiple mating by females: a perspective from quantitative genetics. Animal Behaviour, 35, 939-941.
Herzog, H. A. & Galvin, S. 1997. Common sense and the mental lives of animals: an empirical approach. In: Anthropomorphism, Anecdotes, and Animals (Ed. by R. W. Mitchell, N. S. Thompson, & H. L. Miles), pp. 237-253. New York: SUNY press.
Joy, J. E. & Crews, D. 1988. Male mating success in red-sided garter snakes: size is not important. Animal Behaviour, 36, 1839-1841.
Madsen, T. & Shine, R. G. 1993. Male mating success and body size in European grass snake. Copeia, 1993, 561-564.
Madsen, T. & Shine, R. G. 1994. Costs of reproduction influence the evolution of sexual size dimorphism in snakes. Evolution, 48, 1389-1397.
Madsen, T., Shine, R. G., Loman, J. & Hakansson, T. 1993. Determinants of mating success in male adders, Vipera berus. Animal Behaviour, 45, 491-499.
Noble, G. K. 1937. The sense organs involved in the courtship of Storeria, Thamnophis, and other snakes. Bulletin of the American Museum of Natural History, 73, 673-725.
Perry-Richardson, J. J., Schofield, C. W. & Ford, N. B. 1990. Courtship of the garter snake, Thamnophis marcianus, with a description of a female behavior for coitus interruption. Journal of Herpetology, 24, 76-78.
Pisani, G. R. 1976. Comments on the courtship and mating mechanisms of Thamnophis (Reptilia Serpentes, Colubridae) Journal of Herpetology, 10, 139-142
Rivas. J. A. 1999. Life history of the green anaconda (Eunectes murinus) with emphasis on its reproductive biology. Ph.D. thesis, University of Tennessee, Knoxville.
Semlitsch, R. D. & Gibbons, W. J. 1982. Body size and sexual selection in two species of water snakes. Copeia, 1982, 974-976.
Shine, R. G. 1978. Sexual size dimorphism and male combat in snakes. Oecologia, 33, 269-277.
Shine, R. G. 1993. Sexual dimorphism in snakes. In : Snakes; Ecology and Behavior (Ed. by R. A. Seigel & J. T. Collins), pp. 49-86. New York: McGraw-Hill.
Shine, R. G. 1994. Sexual size dimorphism in snakes revisited. Copeia,1994, 326-346.
Shine, R. G. & Fitzgerald, L. 1995. Variation in matings systems and sexual size dimorphism between populations of Australian python Morelia spilota (Serpentes: Pythonidae). Oecologia, 103, 490-498.
Shine, R. G., Olsson, M. M., Moore, I. T., Lemaster, M. P., Greene, M. & Mason, R. T. 2000. Body size enhances mating success in male garter snakes. Animal Behaviour, 59, F4-F11.
Small, M. F. 1993. Female Choices: Sexual Behavior in Female Primates. Ithaca: Cornell University Press.
Tinbergen, N. 1951. The study of instinct. New York: Oxford University Press.
Tinberlake, W. & A. R. Delamater 1991. Humility, science and ethological behaviorism. The Behavior Analyst, 14, 37-41.
von Uexk�ll, J. 1985. Environment (Umwelt) and the inner world of animals (C. J. Mellor & D. Gove, Trans.). In: The Foundations of Comparative Ethology (Ed. by G. M. Burghardt), pp. 222-245. New York: Van Nostrand Reinhold. (Reprinted from von Uexk�ll, J. [1909] Umwelt and Innenwelt der Tiere. Berlin: Jena).
Weatherhead, P. J., Barry, F. E., Brown, G. P. & Forbes, M. R. 1995. Sex ratios, mating behavior and sexual size dimorphism of the northern water snake, Nerodia sipedon. Behavioral Ecology and Sociobiology, 36, 301-311.
Table 1. Possible benefits and disadvantages of large size in males and females, the benefits and disadvantage of small size can be inferred from the opposite of the reasons mentioned
Table 1. Possible benefits and disadvantages of large size in males and females, the benefits and disadvantage of small size can be inferred from the opposite of the reasons mentioned
|
|
Benefits of large size |
Disadvantage of large size |
|
Both sexes |
1.- Increased number of potential prey species 2.- Ability to subdue prey. 3.- Less frequent feeding on often risky prey 4.- Fewer predators 5.- Lower energetic cost per unit of body mass. 6.- Greater body temperature stability |
1.- More easily detected by predators 2.- Greater energetic needs. 3.- More conspicuous to their prey. 4.- Higher costs of locomotion. |
|
Females Only |
1.- Increased fecundity due to increased coelomic capacity that allows larger clutches. 2.- Possibility of larger offspring with greater chances of survival. |
|
|
Males only |
1.- Increased number of matings and fitness in males if there is male-male physical competition for mating access. |
1.-Higher costs for locomotion and tracking of females during mating season. |

Figure 1.
Breeding female anaconda (Ashely, 475 cm) at the shore of a canal in the
Venezuelan llanos, being courted by 11 males. Click
here to see other pictures of breeding balls

Figure 2. Mating aggregation
of anacondas involving a very large female and 11 males. The female moved out
of the water and dragged with her some of the males that were coiled around her
(A). Other males were removed from their positions and tried to find the female
again to continue courtship. However, some smaller males have mistakenly coiled
around a very large male and are courting him (B).
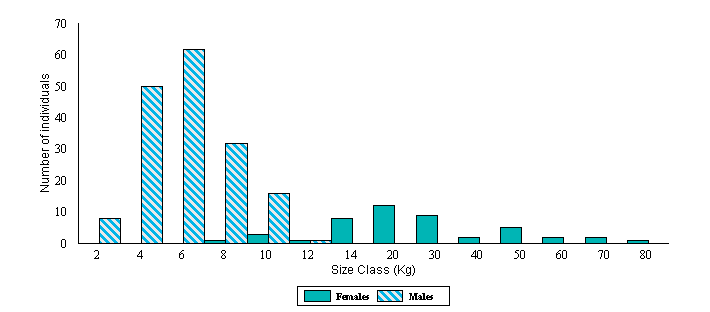
Figure 3.
Size distribution of the adult population of anacondas from the Venezuelan
llanos. The criteria to determine adulthood was finding them involved in a
breeding aggregation. Notice the change in the scale of the "x" axis
after 14 Kg.